December 2007 Edition
plm
Medical device makers find solution to FDA demands
In the medical device industry, efficiently meeting requirements of the Food and Drug Administration is a key to business success, and Product Lifecycle Management (PLM) technology supports FDA compliance integrity by providing comprehensive content to support management decisions across the organization and the individual functional groups. PLM achieves this by uniting the information with the process and resources – people and technology – that enable medical device companies to effectively and economically ensure compliance. The following white paper from Agile Software Corporation explains how.
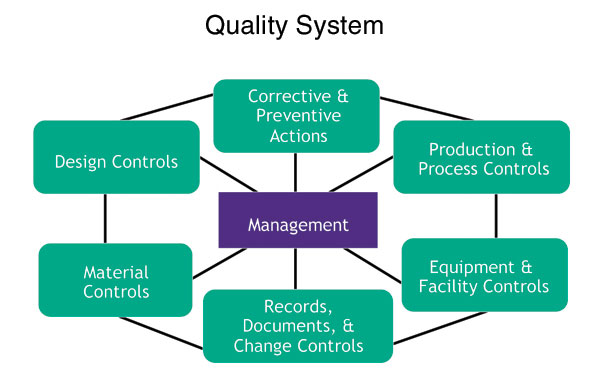
The FDA method for evaluating compliance is the Quality System Inspection Techniques (QSIT). As part of QSIT, the FDA targets six major quality systems that medical device companies must have in place and requires that management demonstrate knowledge on the interrelationships of activities across each of these systems.
QSIT determines compliance by auditing these subsystems and related records. The FDA has become very regimented in looking at these processes and making sure that companies have not only proper procedures to support all these subsystems, but also clear management visibility of any discrete event that happens in any one of these subsystems, and an understanding of exactly how the discrete event impacts the entire organization from a risk-management perspective.
The number of observations from FDA audits continues to increase every year with a significant impact on company performance, so meeting the expectations of the FDA should be a primary concern of any medical device company. Many of the world's top medical device companies have deployed PLM as an indispensable enterprise solution for building an automated compliance process that will meet all the FDA's stringent requirements.
PLM provides a single repository for each of the subsystems audited by the FDA – aggregated across all groups, product lines, and manufacturing sites – providing standardization and accountability of these critical processes across the organization. In addition, PLM provides the necessary management visibility across all these systems, to assess risk management and support better decision-making.
The following outlines each of the six target subsystems, explaining how PLM addresses FDA expectations and delivers associated business benefits.
Corrective and preventive action: CAPA is the key to handling quality events and preventing future quality issues. The FDA expects to see proper handling of a quality event to determine criticality and timely resolution. Managing a quality event adequately requires understanding exactly what documents, product lines, processes, etc., are impacted by the event throughout the organization, identifying what needs to be modified to solve problems caused by the quality event, and following through on corrective action.
Design controls: The FDA requires that a company's design control process provide visibility across all projects and related design activities. Periodic management reviews must be scheduled to verify project status and confirm the requirements have been achieved with documented evidence. Companies must be able to track the origin and verification/validation of design inputs, as well as ensure that original design inputs were manifested in the actual product released to market. The FDA risk-management emphasis also requires processes in place to determine how post-release changes or corrective actions impact original design specifications and controls.
PLM provides a single repository for each of the sub-systems audited by the FDA – aggregated across all groups, product lines, and manufacturing sites – providing standardization and accountability of these critical processes across the organization.
Change controls: The FDA expects all medical device companies to maintain a secure, comprehensive centralized system to manage all quality procedures, product documents and manufacturing procedures, and to track all changes for easy retrieval to support QSIT audit requirements. The FDA also requires that this document-management system enables the company to identify all documents impacted by quality events and product changes.
Material controls: The FDA requires that medical device companies maintain a system to track all materials and associated suppliers used in production, to ensure the quality of those materials and that the final products satisfy design specifications. Companies must demonstrate that they have approved the material supplier and qualified critical materials as part of the development process. Analyzing material cost early in the development process is also critical in achieving price targets for new products. For released product, companies must be able to determine if any quality events are related to material or supplier performance.
Equipment and facility controls: The FDA requires medical device companies to establish and enforce one set of global standard operating procedures for all facility operations worldwide. Companies must also be able to determine if any quality events are related to equipment at a particular facility, and determine all equipment changes necessary to address a quality event.
Production and process controls: Similar to the equipment and facility controls, the FDA requires medical device companies to maintain and enforce one set of production processes across all global manufacturing operations. Companies must be able to identify any quality events related to production processes, manage how quality events and design changes impact manufacturing, and determine all risk associated with production- process changes to justify requalification decisions.
Automating with PLM
Most life-sciences companies currently manage quality and address the QSIT requirements using a combination of manual paper-based processes or discrete-point solutions for document authoring and change management, quality-event management, program management, and management reviews. Each of these four vital business processes can be automated by implementing PLM, delivering dramatic improvements in business performance and compliance to QSIT requirements.
The FDA method for evaluating compliance is the Quality System Inspection Techniques (QSIT). As part of QSIT, the FDA targets six major quality systems that medical device companies must have in place and requires that management demonstrate knowledge on the interrelationships of activities across each of these systems.
Managing documents across the enterprise is a significant challenge, especially for medical device companies that depend on such a wide variety of documents to support compliance.
The existing document management and change process in the life-sciences industry, although well-defined, is often a fragmented manual process, providing no integration with ERP or other enterprise solutions. The process consists of a serial sequence of events that causes lengthy delays and requires non-value-added activity associated with creating, submitting, cross-checking, reviewing, approving, filing, organizing, and implementing change orders across the organization.
Data is all handled manually via lab notes, reporting templates, DHF files located in filing cabinets, hard-copy signatures, PDF documents, and stand-alone non-integrated solutions. Re-entry of information into other systems further increases change order cycle time and opens the potential for errors that can escape the manual validation process.
PLM revolutionizes the process by providing an essential foundation for archiving and sharing product data, managing change, providing secure collaboration with external partners, and automating compliance. PLM supports the creation of a compliant data set consisting of all documents, decisions, and activity to support each of the QSIT systems. Synchronization of this information across each system improves business performance and compliance integrity.
To support document authoring and change management, PLM provides:
- A common database for all enterprise content, including QMS-SOPs, DHF, DMR and regulatory data/records;
- Continuous record of all product data and change history;
- Tracking and management of all revisions;
- Standardized format defined for each functional group;
- Secure document access and management with user roles and privileges (per part FDA CFR part 11);
- Standardized workflows for authoring and revising documents by document type;
- Automated document review and approval process for internal and external collaboration; and
- ERP integration for BOM transfer and ECO cut-ins.
By maintaining a central PLM system for document management and change tracking, a company can significantly boost productivity, cut time wasted on searching for documents, eliminate errors related to use of incorrect or outdated documents, efficiently reuse records, and reduce operations costs.
Quality-event management
The management of quality events – such as CAPA, NCMR, complaints, audits, MDRs, etc. – is supported and processed by the Q/A group, but this quality information may not be effectively aggregated or shared across the enterprise. Without PLM, linking quality and compliance with the supporting records between disparate document and quality-management systems is very difficult, slow, inefficient, and costly.
Consequently, cross-functional investigation of quality events is fragmented; status of event resolution is difficult to monitor; reporting of events is delayed; and quality improvement is difficult to implement. This fosters a reactive corporate culture, instead of a proactive one. That not only compromises business performance, but also impacts regulatory integrity.
The ultimate PLM solution for a medical device company integrates the company's quality-management system with the QSIT compliant dataset to share quality data across the enterprise and automate challenging compliance tasks. Two important capabilities needed for compliance are identifying the source of a quality problem and tracking how quality events impact the organization. PLM provides that vital connection between the quality event and the document trail, demonstrating what caused it, what has been impacted, and what needs to be changed across all systems and processes audited by QSIT.
Managing documents across the enterprise is a significant challenge, especially for medical device companies that depend on such a wide variety of documents to support compliance.
To support quality event management, PLM provides:
- Closed-loop quality event management with root-cause analysis and associated required changes;
- One database, one system, and standard processes to manage all quality events;
- Integration of quality events to DHR/DMR/QMS-SOPs to support risk-management assessment;
- Enterprise visibility of quality-event impact;
- Management of quality investigations in real time through closure; and
- Quality archives to support audit management.
By supporting quality event management in synchronized systems, PLM improves response to quality events, which increases customer satisfaction, notification of regulatory reportable events, product quality, audit integrity – ultimately delivering increased company performance.
Program management
Efforts such as DHF, clinical trials, manufacturing transfer, and CAPA management require formal program management, and must be included in the overall program portfolio analysis for the company. The medical device industry currently faces challenges in assessing program performance and status, forecasting program readiness and completion, and prioritizing resource allocation.
Managing programs across the QSIT subsystems is even more difficult because it is not standardized, creating redundant information, inefficiencies, and errors in the transfer. These errors impact the resolution of quality events and product-development cycle time, and result in clinical and production stoppage, increased unit cost, extended manufacturing transfer times, and significant increases in project cost and compliance risk.
To support program management, PLM provides:
- Standard support for all enterprise projects such as DHF, manufacturing transfers, clinical trials, regulatory submissions, and CAPA management;
- Synchronization of project plans with document and quality system in one database;
- Resource management;
- Tracking and enforcement of project milestones; and
- Direct access to historical DHF content for change-impact analysis and reuse.
Program management via PLM supports improved decision-making to focus resources on projects with the most potential, resulting in faster R&D ROI. Standardization of the DHF process across the organization assures compliance and streamlines the design processes to accelerate cycle times for product development and time-to-market. All of this historical design content provides executive management with an easy reference for risk management and reuse of valuable design IP.
Management reviews
Most medical device companies face the challenges of a communication disconnect between the quality assurance (QA) and business organizations within the company. The first obstacle is a "language barrier" between QA and operations. Reports are often not in a format or language that is easily understood by management, and they are not presented in a compelling way that will call management to action. Consequently, management has difficulty interpreting the business impact quality has on the company and prioritizing appropriate resources to resolve quality issues.
The language barrier between QA and operations is compounded by the fact that quality data is usually presented with text written in QA's jargon, rather than visually, making quality reports difficult for the business people to interpret. The result: senior management does not get the message that quality is a priority.
The second challenge is getting the right information to management. Because of difficulty synchronizing with other functional groups and aggregating data from multiple unconnected systems, QA cannot get its hands on the data it needs, or at least not fast enough. By the time quality information is presented to senior management, the content is outdated. At best, management can only be reactive instead of proactive on quality, spending time mitigating risk rather than strategically leveraging the knowledge to improve the bottom line.
In addition, the messaging aimed at management is incomplete because a disproportionate amount of time is spent gathering the information, leaving no time to analyze the data and make it actionable.
The third challenge is the lack of cross-functional communication flow. When QA-RA processes require cross-functional input with multiple QSIT systems, it often goes into a black hole, with no visibility into status. This disconnect between groups impacts process performance and compliance integrity during audits. Obviously, manual processes make the problem worse, but so do independent automated quality solutions which are not synchronized with enterprise systems used by other functional groups.
All together, these challenges negatively impact company performance, market share, customer satisfaction and even staff morale. To solve the problems, the QA organization needs to present management with a graphic representation of the financial impact of quality – illustrating the cost of recalls, CAPA, inventory, NPI and knowledge lost – supported by an automated system with a common database for all quality information, enabling Quality to gather, share and analyze quality data.
Program management via PLM supports improved decision-making to focus resources on projects with the most potential, resulting in faster R&D ROI.
PLM technology provides a single repository for quality data and the platform to establish a common dialogue and common priorities with Business to foster a compliance culture across the organization. This starts by empowering Quality to get the message out in management's own language. PLM analytics can clarify the issues and provide graphic representations that speak to business people in a language they understand. The information must be presented in real time through clear communication channels, so quality can be visibly tied to the bottom line, to keep management's eye on the ball, and incite it to take action.
PLM can provide the added advantage of analytics that process quality data and translate it into meaningful actionable information. Quality data can be used to help make timely decisions that matter to the business, providing visibility and insight to people across the company.
To support management reviews, PLM provides:
- Automated real-time reporting of all enterprise activity;
- Standard reports for document management, quality events, and projects;
- Custom "dashboards" to present critical information faster;
- Cross-functional analysis of all data in a graphical format; and
- Content synchronization with other enterprise tool such as ERP and MES.
Establishing synchronized management reviews with PLM enables management to make the right decisions to correct quality issues fast, saving money, ensuring customer satisfaction, and protecting market share.
Ideal solution
Life sciences companies need to automate compliance and quality processes to address the QSIT requirements, as well as improve business process performance and compliance integrity. This requires a tool that can automate and synchronize business processes and content management across all functions and locations of the enterprise. PLM is the ideal solution, specifically addressing all FDA expectations with regard to QSIT, as well as enabling medical device companies to improve business performance by streamlining quality and compliance processes, reducing time-to-market, strengthening product quality, reducing cost, and improving response to customer needs.
Agile Software Corporation,
www.rsleads.com/712tp-169
What do you think?
Will the information in this article increase efficiency or
save time, money, or effort? Let us know by e-mail from our
website at www.ToolingandProduction.com or e-mail the editor at
dseeds@nelsonpub.com.